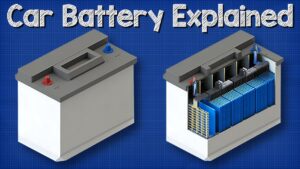
What is a Car Battery? Working of Lead Acid Battery
The Battery is the main part of the electrical system in an automobile. without the battery the engine con not be started with the starting motor. the battery supplies current for operation of the starting motor and ignition system when the engine is being cranked for starting. it also supplies current for light, radio, heater and several other accessory units when the generator is not operating fast enough to handle the electrical load
Lead- acid battery
The lead acid battery is most widely used in automobile
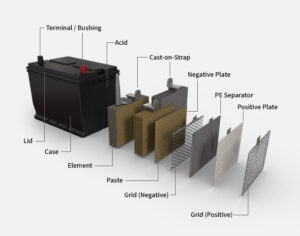
Contraction a lead acid battery consists of the Following components.
- Container
- Plates
- Separators
- Cell covers
- Electrolyte
- Cell connectors
- terminals
Container
The container of a lead-acid battery is a single piece construction and is made of hard rubber plastic or bituminous composition it must withstand extreme of heat and cold as well as mechanical shocks and must be resistant to the absorption of acid it is divided into compartments by partitions for different cells.
Plates
There are two types of Plates Group in each cell positive plate group and negative plate Group The plate group connected to the positive terminal of the cell consists of grids filled with a paste of lead peroxide the plate group connected t the negative terminal on the cell consists of grids filled with metallic lead it is spongy and dull grey in color each group are of the plate is held to gathers by a post strap to which each individual plate is welded these straps extended up through the cell cover to provide the cell terminals to connect one to the other the plate group are arranged in the cell so the so that the positive and negative plates alternate
Separators
Separators are placed between the negative and positive plate to keep them separate between each other the separators are designed to hold the plates apart so that they do not touch and same time they must be porous enough to permit liquid to circulate between the plates separators are usually made of specially treated wood hard rubber resin impregnated fiber alone or in combination with rubber or matsof glass fibers
Cell cover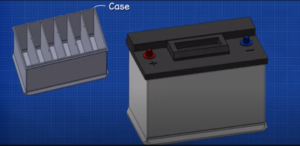
Each cell is sealed by a cover of hard rubber through which the positive and negative terminals project adjacent negative terminals are connector by connector straps each cover has an opening through which liquid can be added . A filler cap is screwed on this opening the filler cap has an air vent for the escape of gas in many late model batteries one-piece cover is provided that covers all the cells
Electrolyte
the electrolyte used in a lead-acid battery is the solution of sulphuric acid. it consists of 40% sulphuric acid and 60% distilled water the level of the electrolyte in the container is about 10mm above the tops of the plate when the electrolyte has been added and the battery is given an initial forming charge it is ready for operation.
Cell connectors
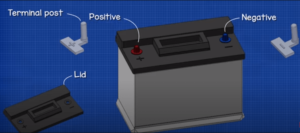
Cell connectors are placed over the protruding terminal parts and welded to them to connect the cells in series connectors must be heavy enough to carry the high current required for starting without overheating
Terminals
Chemical Reaction used for Lead Acid Storage Battery Cells
The main active materials required to construct a lead acid battery are
- Lead peroxide (PbO2).
- Sponge lead (Pb)
- Dilute sulfuric acid (H2SO4).
Lead Peroxide (PbO2)
The positive plate is made of lead peroxide.
Sponge Lead (Pb)
The negative plate is made of pure lead in soft sponge condition.
Dilute Sulfuric Acid (H2SO4)
Dilute sulfuric acid used for lead acid battery has a ratio of water : acid = 3:1.
The lead acid storage battery is formed by dipping lead peroxide plate and sponge lead plate in dilute sulfuric acid. A load is connected externally between these plates. In diluted sulfuric acid the molecules of the acid split into positive hydrogen ions (H+) and negative sulfate ions (SO4 − −). The hydrogen ions when reach at PbO2 plate, they receive electrons from it and become hydrogen atom which again attack PbO2 and form PbO and H2O (water). This PbO reacts with H2 SO4 and forms PbSO4 and H2O (water).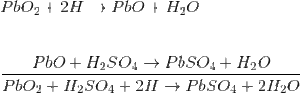
SO4 − − ions are moving freely in the solution so some of them will reach to pure Pb plate where they give their extra electrons and become radical SO4. As the radical SO4 cannot exist alone it will attack Pb and will form PbSO4.
As H+ ions take electrons from PbO2 plate and SO4 − − ions give electrons to Pb plate, there would be an inequality of electrons between these two plates. Hence there would be a flow of current through the external load between these plates for balancing this inequality of electrons. This process is called discharging of lead acid battery.
The lead sulfate (PbSO4) is whitish in color. During discharging,
- Both of the plates are covered with PbSO4.
- Specific gravity of sulfuric acid solution falls due to formation of water during reaction at PbO2 plate.
- As a result, the rate of reaction falls which implies the potential difference between the plates decreases during discharging process.
Now we will disconnect the load and connect PbSO4 covered with PbO2 plate with positive terminal of an external DC source and PbO2 covered with Pb plate with negative terminal of that DC source. During discharging, the density of sulfuric acid falls but there still sulfuric acid exists in the solution. This sulfuric acid also remains as H+ and SO4− − ions in the solution. Hydrogen ions (cation) being positively charged, move to the electrode (cathode) connected with negative terminal of the DC source. Here each H+ ion takes one electron from that and becomes hydrogen atom. These hydrogen atoms then attack PbSO4 and form lead and sulfuric acid.
![]()
SO4− − ions (anions) move towards the electrode (anode) connected with positive terminal of DC source where they will give up their extra electrons and become radical SO4. This radical SO4 cannot exist alone hence reacts with PbSO4 of anode and forms lead peroxide (PbO2) and sulfuric acid (H2SO4).
![]()
Hence by charging the lead acid storage battery cell,
- Lead sulfate anode gets converted into lead peroxide.
- Lead sulfate of cathode is converted to pure lead.
- Terminal; potential of the cell increases.
- Specific gravity of sulfuric acid increases.
Specific Gravity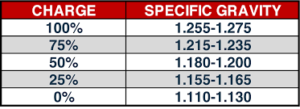
The specific gravity of the electrolyte in a fully charged cell should be from 1.280 to 1.300. If it varies more than 10 points above or below these values, adjust it by drawing off some of the electrolyte with a hydrometer and adding water to lower the gravity, or 1.400 acid to raise the gravity
Hydrometer
The Hydrometer to check the specific gravity in a electrolyte it consist of a glass barrel and bulb syring for sucking up a sample of the electrolyte to float an enclosed glass hydrometer calibrated to read in terms of specific gravity
Electrolyte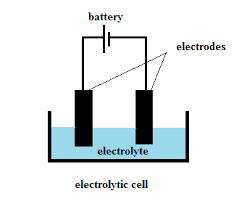
The battery electrolyte is a solution inside batteries. Depending on the type of battery, it can be a liquid or paste-like substance. However, no matter the type of battery, the electrolyte serves the same purpose: it transports positively charged ions between the cathode and anode terminals.
FAQ
Q1: How does a car battery work?
- A: A car battery works by converting chemical energy into electrical energy. It stores electrical energy in the form of chemical reactions and releases it as electrical power to start the engine and power various electrical components in the vehicle.
Q2: What is the basic operation of a car battery?
- A: The basic operation involves a chemical reaction between lead dioxide (positive plate), sponge lead (negative plate), and sulfuric acid (electrolyte). This reaction produces electrical energy that is used to power the car’s electrical systems and start the engine.
Q3: How does a battery generate electricity?
- A: Electricity is generated through a chemical reaction within the battery. When the battery is connected to a circuit, the chemical reaction between the lead plates and sulfuric acid produces electrons, creating a flow of electrical current.
Q4: How does electricity flow in a car battery?
- A: Inside a car battery, electricity flows from the negative terminal to the positive terminal. During discharge (use), electrons flow from the negative lead plate through the external circuit to the positive lead plate, providing power. During charging, this process is reversed.
Q5: What components are involved in a car battery’s operation?
- A: The main components include lead plates (positive and negative), an electrolyte solution (usually sulfuric acid and water), separators, and a casing. These components work together to facilitate the chemical reactions that generate electrical energy.
Q6: How does a car battery start the engine?
- A: The battery supplies electrical energy to the starter motor. When the key is turned, the starter motor engages and cranks the engine. This process requires a significant amount of electrical power, which the battery provides.
Q7: Can a car battery be recharged?
- A: Yes, a car battery can be recharged. When the engine is running, the alternator generates electricity, which is used to recharge the battery. Additionally, external chargers can be used to replenish the battery’s charge when needed.
Q8: What can cause a car battery to fail?
- A: Common factors leading to battery failure include age, extreme temperatures, overcharging, undercharging, and sulfation. Regular maintenance, such as checking the battery’s fluid level and terminals, can help prevent issues.
Q9: How long does a car battery typically last?
- A: The lifespan of a car battery varies but is typically around 3 to 5 years. Factors like climate, driving habits, and maintenance influence its longevity.
Q10: Can a dead car battery be jump-started?
- A: Yes, a dead battery can be jump-started by connecting it to a charged battery or a jump starter using jumper cables. This transfers electrical energy to the dead battery, allowing the vehicle to start.
Engine Parts Names| इंजन के पार्ट्स के नाम
image credit by google image





[…] How a Car Battery Works | Battery system of car […]
[…] How a Car Battery Works | Battery system of car […]