What is petrol or Gasoline?| Definition of Petrol or Gasoline | Gasoline PDF
Definition of Petrol fuel is any substance that can provide heat and produce energy when it is burned. This energy that releases is generally in the form of chemical energy or heat energy.
- Gasoline, also spelled gasoline, also called gas or petrol, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal-combustion engines. It is also used as a solvent for oils and fats. Originally a by-product of the petroleum industry (kerosene being the principal product), gasoline became the preferred automobile fuel because of its high energy of combustion and capacity to mix readily with air in a carburetor.
- Gasoline was at first produced by distillation, simply separating the volatile, more valuable fractions of crude petroleum. Later processes, designed to raise the yield of gasoline from crude oil, split large molecules into smaller ones by processes known as cracking. Thermal cracking, employing heat and high pressures, was introduced in 1913 but was replaced after 1937 by catalytic cracking, the application of catalysts that facilitate chemical reactions producing more gasoline. Other methods used to improve the quality of gasoline and increase its supply include polymerization, converting gaseous olefins, such as propylene and butylene, into larger molecules in the gasoline range; alkylation, a process combining an olefin and a paraffin such as isobutane; isomerization, the conversion of straight-chain hydrocarbons to branched-chain hydrocarbons; and reforming, using either heat or a catalyst to rearrange the molecular structure.
Automobile Fuel Such as gasoline are made mostly of three Elements :-
- Hydrogen (H)
- Carbon (C)
- Oxygen (O)
FUEL = H+C = Hydrocarbon Fuel
Perfects Combustion
- During Combustion Each Oxygen atom Unites with Two Hydrogen atoms oxygen uniting with hydrogen produces H²O or Water
- Carbon Uniting with oxygen produces CO² or the gas carbon dioxide because there are two oxygen atom to each carbon atom
20% Oxygen(O) and 80% Nitrogen (N) with Perfect Combustion
Gasoline = Hydrocarbon +Carbon
Combustion is not perfect
Some of fuel or gasoline(HC) Does not properly Burn Also some only partly this produce (CO) Corban Monoxide (Monoxide only one oxygen atom for each carbon atom)
Fuel Those Combustion Substance Which Produce huge amount of energy after complete combustion are called Fuel.
Harmful Gases
- Co = Corban Monoxide
- No² = Nitrogen Oxides
- HC = Hydrocarbon
Fuel are of two types:-
-
Primary Fuel
-
Secondary Fuel
- Primary Fuel Those combustion substance which are obtained From nature and are directly used Fuel are called primary Fuel Explain – Wood natural Gas
- Secondary Fuel– Those Combustible Substance Which are produced From primary Fuel and used as a Fuel are called Secondary Fuel . L.P.G., Petrol, Diesel, Koil, Wax, Alcohol,

Classification of Fuels
- Solid Fuel
- Liquid Fuel
- Gaseous Fuel
Solid Fuel
These are solid materials that combust to produce energy. Some examples of Solid fuel are coal, charcoal, soot, wood etc. These were most likely the first fuels utilized by mankind. They were the fuels responsible for the invention of fire. Even today they have very widespread household and industrial uses.
Liquid Fuel
These are the fuels we burn to produce mechanical energy and kinetic energy. Most liquid fuels such as crude oil form due to exposure to intense heat and pressure to fossilized remains of plants and animals. Then there are biofuels in liquid form such as ethanol and hydrogen fuel. These fuels are easy to transport and relatively easy to use,
Fuel Gas
Fuel Gas as the name suggests are fuels that are in a gaseous state under normal conditions. Some examples are methane, carbon monoxide, propane etc. They have an advantage that they can be easily transported to the place of consumption. However, they also tend to leak from pipes and every precaution must be taken to avoid this. The best example would be the CNG gas that comes to your kitchen via pipes that you utilize for cooking. This is also known as Domestic fuel.
What is Fuel Rating
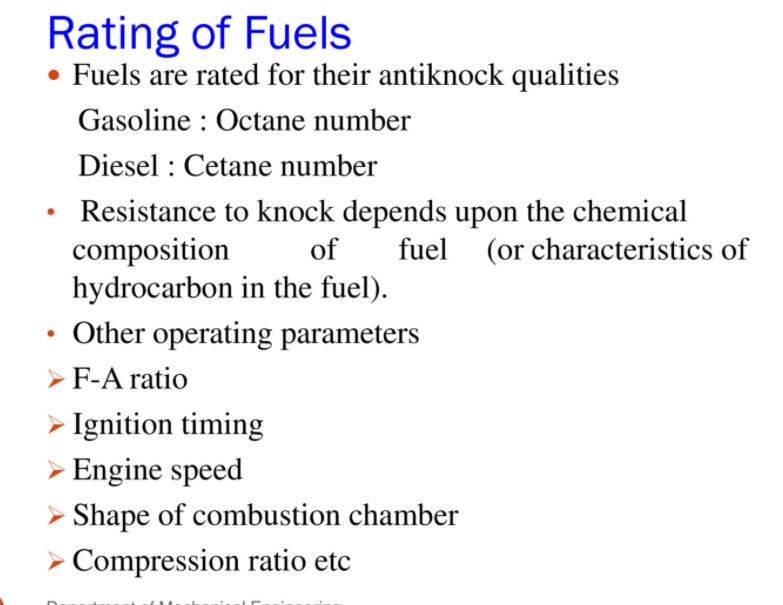
Fuel Rating Fuel used in automobile vehicle differ widely in their ability to resist knock engine knocking is due to severity in operation condition is based upon engine load speed spark advance air-fuel ratio and temperatures different means used to compare the anti knock quality of the fuel standard engine operating under given condition are use to determine fuel rating fuel are generally determined by comparing the performance of standard fuel a standard fuel is combination mixture of iso octane and normal heptane or iso octane and tetra ethyl lead (T.E.L)
Cetane number
cetane number it is the method of rating diesel fuel valve of cetane number of particular diesel fuel is determined by comparing the ignition characteristic of fuel with refence fuel two reference fuel mixture sample of cetane and alphamethy nephelane has poor ignition quality cetane number of sample fuel is the percentage of cetane available in the mixture of cetane and a lphamethyle nepthelena of which ignition quality coincide

Octane number
octane number octane number measure the ability of petrol to resist detonation during combustion actually octane number indicates the antiknock quality of fuel these number was introduced by C.F.R> co-operative fuel research committee in this method of rating two fuel mixture hydrocarbon is used one has worst antiknock property n- heptane and other has best antiknock property iso- octane these two are called reference fuel
n-heptane- o (zero) octane number
iso-octane- 100 octane number
he much the knock characteristic of sample fuel with sample mixture fuel of reference hydrocarbon
About octane ratings
The octane ratings of Synergy™ gasoline
- Synergy™ Regular Gasoline – Octane 87
- Synergy™ Extra Gasoline – Octane 89
- Synergy SUPREME+™ Gasoline – Octane 91-93
What Are the Important Properties of a good Fuel
- Fuel should have High Calorific Value
- It should have optimum ignition Temp
- It Does not produce any harmful gases
- it should be easy to handle and transport
- it does not leave much residue after combustion


